 In the Netherlands we are very proud of our tap water. It is of excellent quality, widely available and incredibly cheap. Due to the reasonably good quality of surface waters and the good quality of the groundwater sources it has always possible to produce tap water with basic technologies like sand filtration and coagulation. However, the impact of human activities on the natural systems is becoming more obvious and we are on the edge of starting to feel the problems. For example the predicted phosphorous shortage, the decrease in natural forests around the world, the decline in polar ice formation, the changes in weather and the increase of waste and anthropogenic compounds we find in our waters. You might have heard about it before, (micro)plastics, pesticides, hormones.
In the Netherlands we are very proud of our tap water. It is of excellent quality, widely available and incredibly cheap. Due to the reasonably good quality of surface waters and the good quality of the groundwater sources it has always possible to produce tap water with basic technologies like sand filtration and coagulation. However, the impact of human activities on the natural systems is becoming more obvious and we are on the edge of starting to feel the problems. For example the predicted phosphorous shortage, the decrease in natural forests around the world, the decline in polar ice formation, the changes in weather and the increase of waste and anthropogenic compounds we find in our waters. You might have heard about it before, (micro)plastics, pesticides, hormones.  Due to these anthropogeneic compounds it becomes a bit more challenging, and perhaps a bit more expensive to make good quality tap water. Several drinking water companies are (considering) switching their form of treatment from the standard methods to more advanced technologies like reverse osmosis (RO) and advanced oxidation. These and other technologies will do perfect at making excellent quality tap water. The energy use and chemical consumption, and hereby the sustainability of these new technologies, will become clear over time. A possible major drawback for the proces of reverse osmosis in the production of a concentrate stream.
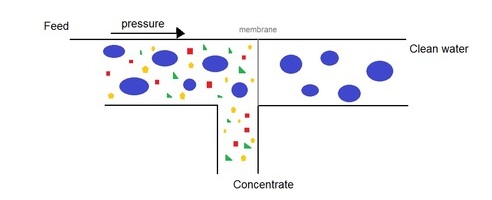
Due to these anthropogeneic compounds it becomes a bit more challenging, and perhaps a bit more expensive to make good quality tap water. Several drinking water companies are (considering) switching their form of treatment from the standard methods to more advanced technologies like reverse osmosis (RO) and advanced oxidation. These and other technologies will do perfect at making excellent quality tap water. The energy use and chemical consumption, and hereby the sustainability of these new technologies, will become clear over time. A possible major drawback for the proces of reverse osmosis in the production of a concentrate stream.
Reverse osmosis:
The dutch water company Evides is investigating if RO concentrate is a showstopper for the use of RO in drinkingwater production. Via the National Watertraineeship I was part of one ot the projectteams asked to answer this question. Our projectteam, including Jordi van Mook, Kyra Huang and Daryl Lue, was assigned to find solutions on what to do with this concentrate. Besides the more standard solutions like ground injection, discharge on surface waters and sea, and discharge via a WWTP we have also looked into more sustainable approaches.
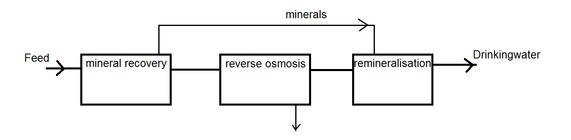
Several design options for a drinking water proces using RO were proposed. From a sustainable perspective these designs were based on the idea of “zero liquid discharge” (meaning you can’t lose a single drop of water), minimizing the amount of chemicals used and minimizing the amount of waste produced (the concentrate). To achieve this it is required to maximize the efficiency of the RO, and minimize the amount of scaling. Usually scaling is minimized by removing minerals from the water using lime or caustic dosing and by adding anti-scalents. After RO a remineralisation step is required to make the water suitable for human consumption. Even though these minerals are the same elements before and after RO (Mg, Ca, etc.), on current designs they are not being recovered and re-used. Wouldn’t it be great if they were?
Recovery of minerals before RO should be done by using a technology which produces a clean mineral product. Available technologies are based on separtion using an electric current (electrodialysis, capacative dionization) or separation based on cristal production (freeze cristallization, cristallization, precipitation). Whether the financial benefits of recovering minerals weighs up with the investment costs of these technologies is something to be seriously considered.
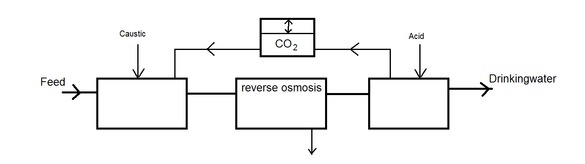
 The different approach does not recover the minerals from the water, but prevents scaling on the membranes by increasing their solubility in the water. For compounds that react with an acid or a base (organic salts) the solubility is dependent of the pH. For some inorganic salts the solubility is indirectly dependent on the pH, for example the precipitation of calciumphosphate is pH dependent due the hydrogenphosphate equilibrium. If the pH of the water is increased before RO the amount of scaling will be minimized. After RO the pH can de reduced to neutral again. Perhaps is is possible to (partially) manage the pH by controlling the carbonic acid equilibrium. Before RO carbon dioxide could be mixed with the water, allowing pH decrease. After RO carbon dioxide could be stripped from the water causing pH increase. This could potentially reduce the amount of chemicals used for pH adjustment.
The different approach does not recover the minerals from the water, but prevents scaling on the membranes by increasing their solubility in the water. For compounds that react with an acid or a base (organic salts) the solubility is dependent of the pH. For some inorganic salts the solubility is indirectly dependent on the pH, for example the precipitation of calciumphosphate is pH dependent due the hydrogenphosphate equilibrium. If the pH of the water is increased before RO the amount of scaling will be minimized. After RO the pH can de reduced to neutral again. Perhaps is is possible to (partially) manage the pH by controlling the carbonic acid equilibrium. Before RO carbon dioxide could be mixed with the water, allowing pH decrease. After RO carbon dioxide could be stripped from the water causing pH increase. This could potentially reduce the amount of chemicals used for pH adjustment.
Dreaming beyond reverse osmosis
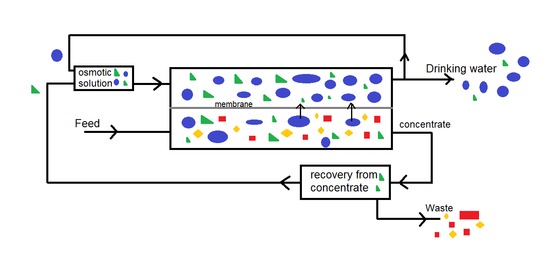
There is a new kid in town. Forward osmosis. Which is basically like reverse osmosis, but reversed. So just osmosis. Revolutionary!! As far as I know it has only been tested on lab scale, but it is showing a lot of potential [1]. The proces is less energy intensive compared to RO, which reduces operational costs. An osmotic solution on the permeate side of the membrane will pull the water through the membrane, leaving behind a concentrated solution. The composition of the osmostic solution can be designed and should be adapted to the quality of the ingoing water. This allows producing drinking water accoring to flavor. The concentrate (from forward osmosis) contains salts, sulphate, nitrate and organic compounds. These components can be recovered and reused in the osmotic solution, which makes the proces economically more attractive.
 I will leave you with some questions. The fact that we cannot drink directly from our groundwater wells or rivers, and we need high-tech solutions to produce drinking water, is that going forward or reverse? And what osmosis technology would be most suited for drinkingwater treatment, are we going with forward or reverse?
I will leave you with some questions. The fact that we cannot drink directly from our groundwater wells or rivers, and we need high-tech solutions to produce drinking water, is that going forward or reverse? And what osmosis technology would be most suited for drinkingwater treatment, are we going with forward or reverse?
Hope to hear from you, Natasja
[1] Critical review of desalination concentrate management, treatment and beneficial use, Pei Xu, State of the art and review on the treatment technologies of water reverse osmosis concentrates, A Perez-Gonzales, Geconcentreerde zoute reststromen BTO 2012.014, september.

Natasja, do you know any product of Evides company available on Amazon or eBay? I don’t see. Thanks
Hi, Evides is one of the Dutch drinking water companies. They only sell water :) this is their website: https://www.evides.nl/paginas/english.aspx best of luck!
I’m researching about reverse osmosis. Nice post, thanks for sharing!
Thank you, best of luck with your research!